Diffusion Tensor Imaging in Autism Spectrum Disorder a Review
- Research
- Open up Admission
- Published:
A improvidence-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-anile children
Periodical of Neurodevelopmental Disorders volume xi, Article number:32 (2019) Cite this article
Abstruse
Groundwork
The core symptoms of autism spectrum disorder (ASD) are widely theorized to result from altered brain connectivity. Improvidence-weighted magnetic resonance imaging (DWI) has been a versatile method for investigating underlying microstructural backdrop of white matter (WM) in ASD. Despite phenotypic and etiological heterogeneity, DWI studies in majority male samples of older children, adolescents, and adults with ASD have largely reported findings of decreased partial anisotropy (FA) across several commissural, project, and association fiber tracts. Nevertheless, studies in preschool-aged children (i.e., < 30–xl months) advise individuals with ASD have increased measures of WM FA before in development.
Methods
We analyzed 127 individuals with ASD (85♂, 42♀) and 54 typically developing (TD) controls (42♂, 26♀), anile 25.1–49.6 months. Voxel-wise effects of ASD diagnosis, sex, age, and their interaction on DWI measures of FA, mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) were investigated using tract-based spatial statistics (TBSS) while controlling mean absolute and relative motion.
Results
Compared to TD controls, males and females with ASD had significantly increased measures of FA in eight clusters (threshold-costless cluster enhancement p < 0.05) that incorporated several WM tracts including regions of the knee, torso, and splenium of the corpus callosum, inferior frontal-occipital fasciculi, inferior and superior longitudinal fasciculi, middle and superior cerebellar peduncles, and corticospinal tract. A diagnosis past sex activity interaction was observed in measures of Advertizement beyond half dozen significant clusters incorporating areas of the torso, articulatio genus, and splenium of the corpus collosum. In these tracts, females with ASD showed increased Ad compared to TD females, while males with ASD showed decreased Advertizing compared to TD males.
Conclusions
The current findings support growing evidence that preschool-aged children with ASD have singular measures of WM microstructure that appear to differ in directionality from alterations observed in older individuals with the condition. To our knowledge, this written report represents the largest sample of preschool-anile females with ASD to be evaluated using DWI. Microstructural differences associated with ASD largely overlapped between sexes. However, differential relationships of AD measures indicate that sexual activity likely modulates ASD neuroanatomical phenotypes. Further longitudinal study is needed to ostend and quantify the developmental relationship of WM structure in ASD.
Background
The cadre symptoms of autism spectrum disorder (ASD), i.e., deficits in social communication, social interaction and repetitive and restricted behaviors [1], are widely theorized to outcome from contradistinct brain connectivity [2,3,4,v]. Magnetic resonance imaging (MRI), particularly diffusion weighted MRI (DWI), has been a versatile method for investigating underlying microstructural properties of WM in ASD in vivo. Several DWI studies accept reported that individuals with ASD have atypical improvidence backdrop within commissural, association, and project fiber tracts [half-dozen,vii,eight] which are likely to reflect altered neural connectivity. Withal, to appointment most of these studies take included majority male samples of older children, adolescents, and adults. In dissimilarity, the relatively few studies that include preschool-aged children (i.e., < 50 months) propose individuals with ASD have increased measures of WM FA earlier in life [nine]. Furthermore, certain subgroups, e.chiliad., females with ASD, remain understudied and thus associated WM neuroanatomical phenotypes in these groups remain poorly understood.
Altered neural connectivity in ASD was first proposed in terms of deficits in "long"-range connectivity combined with associated hyper "short"-range connections [2, 4]. However, a recent review of functional connectivity studies suggests that altered neural connectivity in ASD may be better understood in terms of network and/or task specific over and under connectivity [5]. In add-on to functional evidence, a large body of work suggests that individuals with ASD have atypical WM construction indicative of altered structural connections. For example, significant increases in WM volumes have been observed in young children and adolescents with ASD compared to typically developing (TD) controls [ten] while the corpus callosum, the largest WM fiber parcel in the brain, has been extensively studied and found to have both atypical morphology and diffusion properties in ASD [7, eight, 11,12,13]. Furthermore, limited postmortem show suggests that adults with ASD accept increased numbers of thin prefrontal axons with reduced myelin density [xiv].
In efforts to categorize WM alterations in ASD, DWI has been particularly valuable for its ability to investigate microstructural properties of WM tracts in vivo. Most commonly, DWI studies assess the anisotropic diffusion backdrop characteristic of WM through tensor-based indices such as fractional anisotropy (FA) and mean diffusivity (Medico) [15, 16], which have been related to several axonal backdrop including bore, packing density, fiber orientation, tortuosity, membrane permeability, and myelin content [17,18,19,twenty]. More specific measures (i.e., axial (Ad) and radial (RD) diffusivity) quantify diffusion parallel and perpendicular to the principle direction of diffusion and thus may assist in interpreting axonal properties (e.g., myelination, fiber loss) which may alter anisotropic improvidence [21].
To date, DWI studies of ASD have typically included more often than not male samples of older children, adolescents and adults. For example, just 5 of 59 ASD DWI studies highlighted in a review of the literature by Ameis and Catani [vi] report an ASD sample with a hateful age beneath 5 years [22,23,24,25,26] and but 2 report samples including at least 10 ASD females [25, 27]. The large majority of DWI studies of older bulk male samples which utilize tensor-based metrics have reported findings of decreased FA across several commissural, projection, and clan fiber tracts, many of which have been linked to social and chatty functioning [6, 7, 11, 28,29,30,31,32,33,34,35,36,37,38,39,xl,41]. However, dynamic interrelationships between brain construction and function provide a challenge in determining the underlying etiology of atypical neural connectivity in ASD based on measures gathered later in life and should be considered from a developmental perspective [42].
Accordingly, studies of early evolution are critical for understanding how atypical brain structure and connectivity contribute to later on ASD phenotypes. Compared to studies in older individuals, relatively few DWI studies have focused on preschool-aged children (i.east., < 30-40 months). Results from these studies suggest WM neurophenotypes in ASD are characterized by increased FA earlier in development [22, 26, 43,44,45,46]. To appointment the large bulk of MRI studies in ASD take included relatively pocket-sized sample sizes (east.g., 10-20 individuals) often spanning a wide age range and multiple developmental stages (eastward.g., babyhood, late childhood, adolescence, and machismo). Such sampling limitations open upward the possibility of averaging out and/or beingness underpowered to detect developmental effects. Furthermore, it is of import to note that (on average) MRI samples of older individuals with ASD may differ in phenotypic severity than those in young children as nocturnal slumber protocols [47] permit for scanning of more severely affected individuals with ASD that are likely to not tolerate the nature (e.k., loud, claustrophobic) and demands (e.g., laying still for long periods of time) of MRI while awake.
Females with ASD have likewise been largely underrepresented in enquiry studies. Identifying sex differences associated with ASD is critical as show suggests that ASD females may accept distinct phenotypes from males and that factors associated with sexual practice may modulate ASD liability (e.g., "female person protective" and "male take chances" models) [48]. Within TD, emerging research indicates the existence of sex differences in the structural connectome [49, l]. Such differences stand for 1 potential sexual activity factor which could contribute to pregnant sex-by-ASD diagnosis effects that take been reported in WM structure [51,52,53]. Within preschool-aged samples, studies of sex differences in tensor-based metrics are limited and have included relatively pocket-sized sample sizes (e.g., due north = 7–13 ASD females) but seem to suggest similar relationships of increased FA in ASD across sexes [44, 45]. Thus, in club to determine if DWI findings in ASD are replicable in samples that more accurately stand for the diversity of the autism spectrum in terms of severity and beyond sexes, additional research is needed.
In the current study, nosotros sought to characterize WM diffusion properties associated with ASD in a sample of male and female preschool-anile children. We utilize DWI acquired during natural nocturnal slumber [47] to investigate measures of FA, Physician, RD, and Advertising beyond whole encephalon WM using a voxel-wise tract-based spatial statistics (TBSS) approach [54]. We hypothesize that individuals with ASD will have significant differences in WM diffusion backdrop in tracts previously indicated in the condition, including the corpus callosum and superior longitudinal fasciculus. To our noesis, our study represents the largest diffusion imaging study in terms of inclusion of preschool-aged females with ASD. Based on prior DWI findings from our group reporting significant sexual practice differences in TD [55] and diagnosis-by-sex interaction effects in ASD [52], nosotros conceptualize both a significant main issue of sex and diagnosis-past-sexual activity interactions in diffusion measures.
Methods
Participants
We analyzed a cross exclusive sample of 127 individuals with ASD (85♂, 42♀) and 54 typically developing (TD) controls (42♂, 26♀), ages 25.1–49.6 months (Tabular array 1). Participants were enrolled in either the ongoing UC Davis Medical Interventions in Neurodevelopmental Disorders (MIND) Plant longitudinal Autism Phenome Project (APP) or Girls with Autism: Imaging of Neurodevelopment (Gain) studies. The design of these studies involves enrolling and conducting baseline MRI in children at 24–42 months of age and and then imaging at almanac intervals for two additional time points. The electric current cross sectional study sample included all individuals in the APP/Proceeds cohorts below the age of 50 months who had successfully completed structural, diffusion-weighted, and phase-mapping MRI scans post an MRI scanner upgrade in August 2009. Previous DWI studies that have utilized subgroups of the currently described sample included data acquired both prior to and later on this upgrade [52, 55]. In cases where participants had successfully completed scanning at more than one fourth dimension betoken prior to l months, data from their get-go (i.e., youngest) bachelor time indicate was always used.
All participants were required to exist native English language speakers, ambulatory, take no contraindications for MRI, no suspected vision or hearing problems or known genetic disorders or neurological weather. An ASD diagnosis was confirmed at study entry past trained clinical psychologists using the Autism Diagnostic Observation Schedule-Generic (ADOS-Grand) [56] or ADOS-ii [57], the Autism Diagnostic Interview-Revised (ADI-R) [58] and DSM-Iv-TR criteria [ane]. Based on their scores on these measures, participants were included according to criteria for young children with ASD established past the Collaborative Programs of Excellence in Autism network. Every bit specified past these criteria, all ASD participants met ADOS-2 cutoff scores for either autism or ASD. In improver, they exceeded ADI-R cutoff scores for autism on either the social or communication subscale and were inside 2 points of this criterion on the other subscale. ADOS-calibrated severity scores were calculated to allow comparison of autism severity across participants tested with different ADOS modules [59]. At Time 1, TD individuals were screened for autism traits using the Social Communication Questionnaire (SCQ) (i.e., scores beneath 11) [lx] and were required to have no beginning-degree relatives with an ASD diagnosis.
The Mullen Scales of Early Learning (MSEL) [61] was used to assess developmental quotient (DQ) during participants start visit (Time 1). TD children were excluded if they did not fall within two standard deviations on the MSEL. MRI information from the second visit (Fourth dimension ii) for 17 participants' (northward = 11 ASD♂, iv TD♂, one ASD♀, i TD♀) was used due to quality bug with or failure to learn their Fourth dimension one MRI data. For these 17 participants, nosotros report MSEL, ADOS, and ADI scores from their first visit. All aspects of the study protocol were approved by the University of California, Davis Institutional Review Board, and informed consent was obtained from the parent or guardian of each participant.
Prototype conquering
All MRI scanning was performed at the Imaging Inquiry Center, UC Davis, Sacramento, during natural nocturnal sleep without sedation [47] from October 2009 to July 2018, using a 3-T Siemens Magnetom Trio MR system (Erlangen, Germany) with an eight-channel head gyre. High-resolution T1 images were caused using an MPRAGE sequence (1 mm3 resolution, TR = 2170 ms, TE = four.86 ms, TI = 1100 ms, FA = 7°, 192 slices, 256 × 256 × 192 mm FOV). Diffusion-weighted images (DWI) were acquired in thirty independent directions forth with five interleaved not-diffusion weighted (b = 0) images (one.9 mm3 resolution, TR = 8500 ms, TE = 81 ms, b = 700, echo spacing = 0.69 ms, GRAPPA iPAT factor = ii, 72 slices, 243 × 243 × 137 mm FOV). An accompanying phase map image was acquired using the aforementioned shim equally the DWI sequence to correct for field inhomogeneities (4 mmthree resolution, TR = 1000 ms, TE = 3.60/6.06 ms, FA = 90°, 48 slices, 256 × 256 × 230 mm FOV).
Diffusion-weighted image preprocessing
Diffusion-weighted images were preprocessed using the MRtrix3 package (www.mrtrix.org) which utilizes elements of the FSL ([62]; fsl.fmrib.ox.air-conditioning.united kingdom) diffusion toolbox (e.g., "eddy" [63]). Preprocessing steps included (ane) paradigm denoising according to a principle component analysis-based method [64, 65], (2) Gibbs ringing artifact reduction [66], (3) correction for distortion due to eddy currents and betwixt volume movements using FSL'due south eddy tool [63] with the options to (4) replace slices with average intensity at least four standard deviations lower than the expected intensity with an interpolated Gaussian process prediction [67], and perform (5) within volume (i.e., slice to book) motion correction [68], the latter of which utilizes the NIVIDA CUDA parallel computing platform (programmer.nvidia.com/cuda-zone). (six) Individual field map images were then calculated and used to right for field distortions while simultaneously registering the improvidence images to their corresponding T1-weighted image using FSL epi_reg [69,70,71]. (7) Lastly, all preprocessed volumes were visually screened by the kickoff writer to insure quality of betwixt book registration and to identify potential image misorientation, slice dropout, and distortion effecting WM regions.
Head motion
Prototype artifacts associated with caput motion are a significant confound in ASD research. Head motion has shown to be increased in ASD [72] and to significantly bear upon DWI results [73]. Accordingly, in addition to utilizing a noctoral slumber protocol [47] and country of the art inside book motion correction [68], we quantified head motion using the root-mean-square (RMS) displacement of both the hateful accented intervolume deportation with respect to the first image of each conquering and the mean relative intervolume displacement between each preceding image in the sequence. Participants with a mean absolute RMS displacement greater than 1.0 mm (n = 4 ASD♂, 0 TD♂, ii ASD♀, 1 TD♀) were excluded from farther analysis and are not described in this written report. For all other participants, mean absolute and relative RMS displacement across volumes were included as covariates in all further analyses.
Diffusion tensor modeling and tract-based spatial statistics
Diffusion was modeled by the fitting of a tensor at each voxel using FSL's diffusion toolbox. Each tensor can be divers past its three principle Eigen vectors (i.e., λ i, λ 2, λ iii). Tensor maps were used to calculate corresponding maps of fractional anisotropy (FA; \( \sqrt{\frac{{\left({\lambda}_1-{\lambda}_2\correct)}^2+{\left({\lambda}_2-{\lambda}_3\correct)}^2+{\left({\lambda}_1-{\lambda}_3\right)}^2}{2\left({\lambda}_1^2+{\lambda}_2^2+{\lambda}_3^two\right)}} \)), mean diffusivity (Dr.; (λ 1 + λ 2 + λ 3)/3), radial diffusivity (RD; (λ two + λ three)/2), and centric diffusivity (AD; λ 1).
Whole-encephalon voxel-wise statistical analysis of FA, MD, RD, and AD maps was conducted using tract-based spatial statistics (TBSS) [54]. Commencement, BET encephalon extraction [74] was performed on each FA paradigm and cease slices zeroed to remove probable outliers from the tensor plumbing fixtures. A study-specific template was so derived by registering each individual'south FA prototype to all other FA images (i.e., tbss_2_reg -due north). The image establish to exist most representative of the sample (i.due east., target image) was then affine-aligned into MNI152 standard space. All FA images were and then registered to MNI152 past combining the nonlinear transform to the target image with the affine transformation of the target to MNI152 infinite. A mean FA image of all participants was then used to derive a white affair "skeleton" which was thresholded to include FA values > 0.two. This resulting white matter skeleton was so used as a binary mask on which individual's measures of FA, MD, RD, and AD were separately projected and subsequently exported for voxel-wise statistical analysis.
Statistical analyses
Non-parametric statistical inference of voxel-wise TBSS-skeletonized measures of FA, Doc, RD, and AD were estimated by regression of a general linear model using FSL's "randomise" [75]. Diagnostic group and sex were included as categorical factors with historic period in months, mean absolute, and relative movement as continuous covariates:
$$ {Y}_i={\beta}_0+{\beta}_1\mathrm{Diagnosis}+{\beta}_2\mathrm{Sexual practice}+{\beta}_3\mathrm{Age}+{\beta}_4\mathrm{absMove}+{\beta}_5\mathrm{relMove}+{\varepsilon}_i $$
where ε i is the residual error at voxel i. Diagnosis-by-sex (β 1Diagnosis∗β 2Sexual practice), diagnosis-past-historic period (β 1Diagnosis∗β threeAge), and sexual activity-by-age (β 2Sex∗β 3Age), interaction effects were tested by adding these terms separately to the above model. Diagnosis-by-sex-by-age (β 1Diagnosis∗β twoSex∗β iiiAge) interaction furnishings were tested for by calculation this and the lower order ii-way interaction terms to the above model. Statistical thresholding and correction for multiple comparisons was conducted via a threshold-costless cluster enhancement (TFCE) [76] permutation (n = ten,000) image to identify meaning (p < 0.05) effects of diagnosis (β one), sex (β two), age (β 3), and the higher up interaction terms for each DWI measure.
Results
Participant demographics
Across the entire sample (i.e., males and females), individuals with ASD were found to exist significantly younger than TD controls (t = 2.45, p = 0.01). This issue was driven primarily past a significant departure in age betwixt ASD and TD males (t = two.72, p = 0.008) which was not observed between ASD and TD females (t = 2.45, p = 0.45). Across diagnostic groups, males did non significantly differ in historic period from females (t = − 0.53, p = 0.59). Equally expected, individuals with ASD had significantly lower MSEL DQ scores than TD participants (t = − 12.55, p = <0.001). Beyond diagnostic groups, no significant divergence in MSEL DQ was constitute between males and females (t = − 1.52, p = 0.12). No significant differences in ADOS severity scores, ADI social, behavior or communication measures were observed between males and females with ASD diagnoses (p > 0.05). No significant differences between diagnostic groups or sexes were observed for mean absolute or mean relative RMS motion parameters (p > 0.05). See Table one for participant demographics.
Diagnostic grouping differences in white matter diffusion backdrop
Voxel-wise assay showed individuals with ASD compared to TD controls had significantly (TFCE p < 0.05) increased FA in eight clusters that incorporated several white matter tracts including regions of the corpus callosum, corona radiata, and inferior and superior longitudinal fasciculi every bit well as the eye and superior cerebellar peduncles, and corticospinal tract (Fig. ane, Tabular array two). Within all viii clusters, increased FA in ASD was observed across sexes, i.eastward., increased FA in ASD was non sexual activity-specific (Fig. two). No clusters exhibiting significant (TFCE p < 0.05) between grouping differences were observed for measures of MD, RD, or AD.
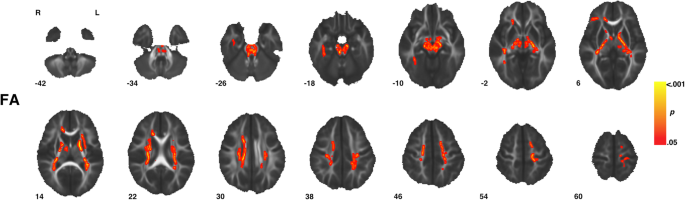
Regions of increased fractional anisotropy in ASD. Individuals with ASD diagnoses showed significantly (TFCE p < 0.05) increased measures of fractional anisotropy (FA) across viii clusters (Table two) highlighted above. Indicated white affair tracts include regions of the corpus callosum, corona radiata, and inferior and superior longitudinal fasciculi besides as the centre and superior cerebellar peduncles and corticospinal tract. Images are presented in R/L radiological convention with MNI z coordinates in millimeter. Skeletonized statistical overlays accept been "inflated" for display
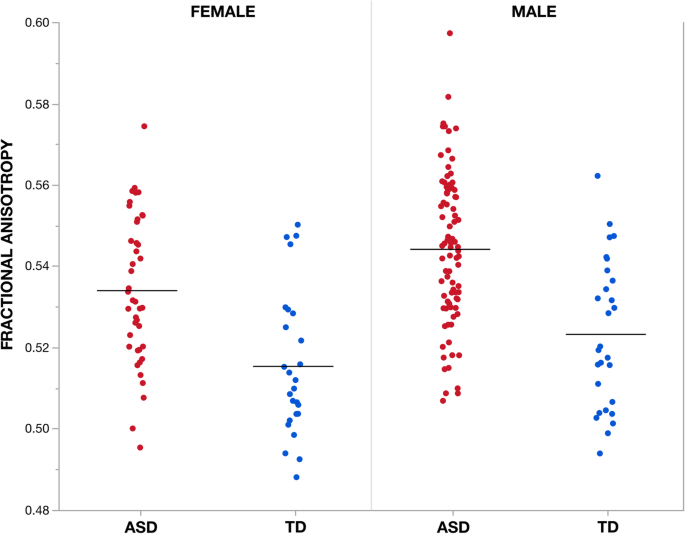
Effect of group on fractional anisotropy measures beyond individuals. Mean partial anisotropy (FA) measures within the largest cluster (i.east., cluster i) showing a significant (TFCE p < 0.05) effect of group (ASD > TD) are plotted for each individual according to group and sex. Cluster 1 incorporates bilateral regions of the middle and superior cerebellar peduncles, corticospinal tract, cerebral peduncle, and internal capsule as well as left corona radiata, thalamic radiation, external capsule, fornix, superior longitudinal fasciculus and fronto-occipital fasciculus. Of note, both males and females with ASD diagnoses testify increased FA compared to TD males and females
Main effects of historic period and sex in white affair improvidence properties
Voxel-wise analysis showed a significant (TFCE p < 0.05) main issue of historic period for all children (i.e., across both diagnostic groups and sexes) in all four improvidence measures in expansive overlapping clusters that incorporated a bulk of all white matter tracts (Additional file 1: Figure S1, Boosted file three: Table S1). Increased FA with age was accompanied past decreased Md, RD, and Advertising in these clusters. Like trajectories of increased FA with historic period were observed across sexes and groups (Fig. 3).
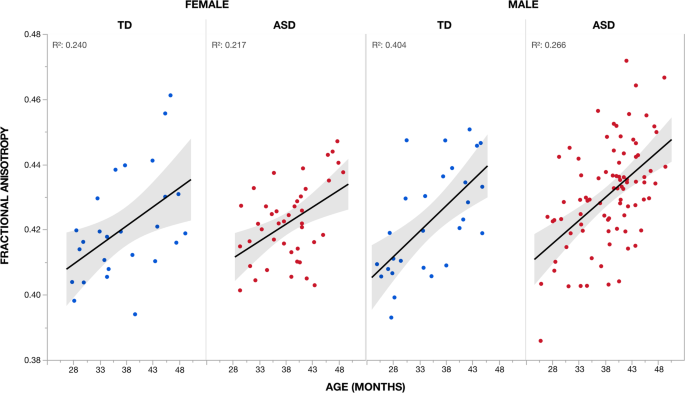
Effect of age on fractional anisotropy across individuals. Mean fractional anisotropy (FA) measures for the cluster (Additional file 3: Tabular array S1) showing a significant (TFCE p < 0.05) positive effect of age are plotted for each private according to group and sex. This cluster incorporated a bulk of all white affair tracts (Additional file 1: Figure S1). Increases in FA with age were observed across both groups (i.e., ASD and TD) and sexes (i.eastward., male and female). Coefficients of determination (R 2) for goodness of fit are provided. Shaded region indicates 95% confidence interval
Furthermore, beyond diagnostic groups, males were found to accept significantly (TFCE p < 0.05) increased measures of FA compared to females across half dozen clusters that incorporated a majority of all white matter tracts. Overlapping significant decreases in Doctor and RD were observed in several of these tracts, merely were absent in some posterior tracts including the posterior thalamic radiations, forceps major, and retrolenticular role of the internal capsule (Additional file 2: Figure S2, Boosted file 3: Tabular array S2). No clusters showing meaning effects of sex were found for measures of Advertisement.
Interaction furnishings between diagnosis, sex, and age in white matter diffusion properties
Voxel-wise assay showed no significant (TFCE p < 0.05) diagnosis-by-age, sex-by-historic period, or diagnosis-by-sex-past-age interaction furnishings beyond all iv diffusion measures. Yet, significant diagnosis-by-sexual activity interactions were observed in measures of AD across vi clusters incorporating areas of the body, genu, and splenium of the corpus collosum too equally areas of the right corona radiata and external capsule (Fig. 4, Table two). Within these regions ASD males showed decreased Advertizing compared to TD males while ASD females showed increased Advertizement compared to TD females (Fig. five). No pregnant (TFCE p < 0.05) diagnosis-by-sex activity interaction effects were observed for measures of FA, Dr., or RD.
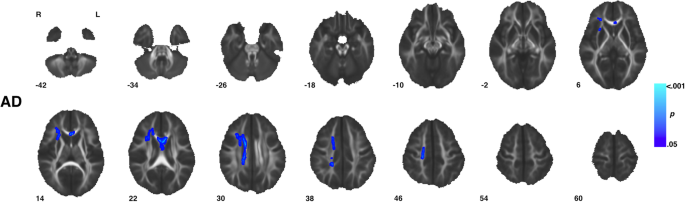
Regions with grouping by sexual activity interaction in axial diffusivity. Clusters (Table 2) showing a significant (TFCE p < 0.05) group past sexual practice interaction effect in measures of axial diffusivity are highlighted. In full, six clusters incorporated areas of the body, genu, and splenium of the corpus collosum too equally areas of the right corona radiata and external capsule. Within these regions, ASD males showed decreased AD compared to TD males while ASD females showed increased AD compared to TD females (Fig. 7). Images are presented in R/Fifty radiological convention with MNI z coordinates in millimeters. Skeletonized statistical overlays accept been "inflated" for display
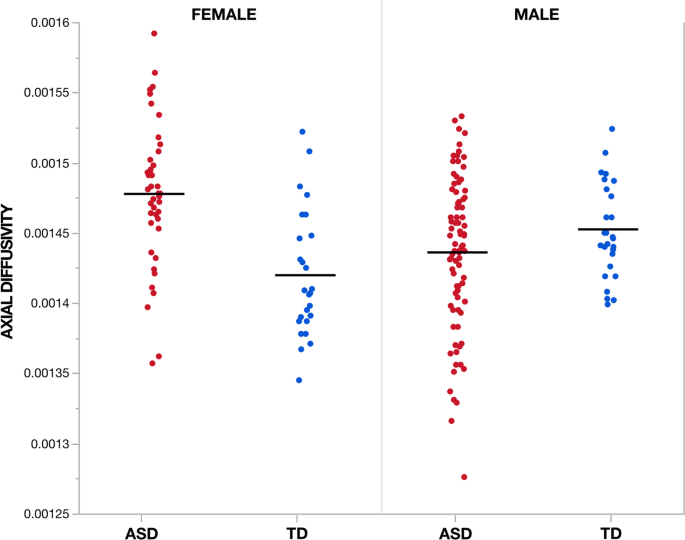
Grouping by sex interaction effects in centric diffusivity across individuals. Private'southward hateful centric diffusivity (AD) measures are plotted co-ordinate to group and sex for the largest cluster (1) for which a meaning (TFCE p < 0.05) grouping by sex interaction effect was observed. Cluster 1 incorporates regions of the genu and body of the corpus callosum as well equally the right inductive and superior corona radiata. Across all six clusters, ASD males showed decreased Advertizing compared to TD males while ASD females showed increased AD compared to TD females. Units for measures of Advertizement are given in mm2/south
Discussion
Our aim was to characterize WM structural backdrop associated with ASD in preschool-anile children using a whole-encephalon, voxel-wise DWI approach. Nosotros constitute that individuals with ASD had significantly increased measures of FA compared to TD controls within several commissural, association, and project WM tracts. While both males and females with ASD demonstrated increased FA, significant sex-by-diagnosis interactions in measures of AD indicate that sex differences modulate WM neuroanatomical phenotypes in ASD. Caution must exist taken in interpreting altered anisotropic diffusion properties as directly reflecting increased or decreased connectivity in ASD [78]. However, these findings support growing show that young children with ASD accept atypical measures of WM microstructure [nine, 22, 26, 43,44,45,46] that may contribute to core ASD symptomatology and differ in directionality from alterations observed in older children, adolescences and adults with the condition [vi, seven, 11, 28,29,30,31,32,33,34, 36,37,38,39,40,41].
Of the WM tracts identified as having increased measures of FA, the corpus callosum is the about widely studied and implicated in ASD [seven, 8, 13]. This tract provides extensive long-range connections in the brain and has been implicated in social and chatty functioning [79]. Within ASD, individuals accept been shown to have smaller callosal volumes [12, 13, 52] and reduced interhemispheric functional connectivity suggestive of deficits in commissural tract integrity [80]. We also identified increased FA inside the inferior longitudinal and inferior frontal-occipital fasiculi. Both of these tracts accept been indicated in prior DWI studies of ASD [vi, 7, 33,34,35, 37] and have shown to be important in the recognition of emotional facial expressions [81]. Of note, the largest cluster of increased FA in the electric current study incorporated the middle and superior cerebellar peduncles. While classically associated with motor coordination [82], recent evidence suggests that the cerebellum plays a disquisitional role in the adaptive control of cortical processing [83] and has been linked to the establishment of normative social behaviors in preclinical models of ASD [84]. Postmortem studies of ASD have noted singular Purkinje cell density in the cerebellum [85, 86] indicating early disruption of cerebellar evolution in the status. Recently reported singular expression of oligodendrocyte-specific genes in the cerebellum of individuals with ASD highlights one potential pathway towards altered cerebellar development and myelination in the condition [87]. Collectively, the electric current observation of singular measures of WM microstructure and/or fiber orientation within these tracts appears likely to reflect atypical neural connectivity associated with ASD.
These findings support a growing body of evidence that indicates immature children with ASD have increased FA compared to TD controls [nine, 22, 26, 43,44,45,46]. Given ASD probable manifests prenatally [88] and is starting time clinically diagnosable around two years of age, early-life measures of brain structure and connectivity not only are critical to agreement the biological basis of autism but also demand to be considered from a developmental perspective [42]. To appointment a large majority of DWI studies have reported atypical measures of WM microstructure in older children, adolescents, and adults with ASD in the form of decreased FA, ofttimes accompanied by increased Physician, in WM tracts implicated in social functioning [6, 7, 11, 28,29,thirty,31,32,33,34,35,36,37,38,39,40,41]. Based on previous findings, the transition from increased FA in younger children with ASD to observed decreases afterward in life appears to manifest onetime between 30 and 40 months of age [nine, 44], suggesting WM undergoes an atypical developmental trajectory in ASD.
Our study focused on a cross sectional sample and is thus non able to directly address hypotheses relating longitudinal changes. However, the historic period range of the current cohort (~ 20–50 months) does capture the menstruum of development when increased FA would exist hypothesized to transition to decreased FA in the condition. Within our accomplice, across both groups and sexes, we observed increased FA and decreased MD, RD, and Advertizing with age across a big majority of all WM tracts. Nosotros did not notice pregnant diagnosis-past-age effects. Thus, our findings practise non suggest a differential developmental trajectory in measures of improvidence properties associated with ASD across the age range of our sample (i.e., ~ xx–50 months). This is in contrast to two studies that have tracked DWI measures in ASD longitudinally prior to fifty months of age, albeit in relatively small samples, that propose that early increases in FA later develop into decreased FA in ASD [44, 45]. Appropriately, the current study highlights the need for boosted longitudinal investigations of WM structure to fully categorize the developmental relationships of DWI measures in ASD across early evolution and into middle childhood, adolescence, and adulthood.
To our cognition, this study includes the largest DWI sample of preschool-aged females with ASD. This is important as females are largely underrepresented in ASD research and may have differences in both behavioral and neuroanatomical phenotypes from males with the status [48]. Beyond diagnostic groups, we observed a significant chief effect of sex characterized by increased FA and accompanying decreased MD and RD in males compared to females beyond a majority of all WM tracts. The global nature of these sex effects suggests a mediating role of differential sexual processes (eastward.1000., steroid hormones) during early development on WM microstructure [89]. Findings of increased FA in males accept been reported by others [xc, 91] as well equally by a previous study that included a portion of the TD command participants currently described [55]. Within the current study, both males and females with ASD showed like relationships of increased FA compared to TD controls in the tracts described in a higher place. Withal, we did find a pregnant diagnosis-by-sex interaction in measures of AD mainly inside the genu and body of the corpus callosum as well as anterior and superior regions of the corona radiata. Inside these clusters, females with ASD showed increased Advertizement compared to TD females, while males with ASD had decreased Advertising relative to TD males. Differences in AD betwixt ASD and TD were also larger in females than males. This outcome is similar to a prior study from our group that identified increased Ad, RD, and Doctor in the corpus callosum of females with ASD but not males compared to TD controls [52]. Every bit AD quantifies the principle direction of diffusion within a voxel, of the currently studied measures of improvidence anisotropy, Advertisement is probable to exist particularly sensitive to overall fiber orientation. Thus, the current finding may reflect an interaction of TD sex differences in the structural arrangement of WM connections [49, fifty] and sex differences associated with ASD neuroanatomical phenotypes [52]
Conclusion
Findings of increased FA in preschool-aged children with ASD suggest that altered WM structural properties are evident in ASD at an historic period when current diagnostic cess of the condition is commencement possible and that these differences are likely to exist reflective of singular neural connectivity. Like differences in WM microstructure were observed in both ASD males and females, although differential relationships of measures of AD betwixt sexes betoken a mediating role of sex in WM microstructure and/or cobweb orientation in the condition. We did not detect bear witness of unlike age-related furnishings in DWI measures between groups within our cross exclusive sample. This study represents a primary analysis to characterize WM structural properties in a subsample of children under l months of age. A follow up longitudinal study will be required to confirm and quantify the developmental relationship of WM structure in ASD and beyond sexes.
Availability of data and materials
Data described in the current report is bachelor from the corresponding writer on reasonable request.
References
-
American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub.
-
Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. Journal of Neuroscience. 2004;24(42):9228–31.
-
Only MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neuroscience & Biobehavioral Reviews. 2012;36(4):1292–313.
-
Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(8):1811–21.
-
Picci Yard, Gotts SJ, Scherf KS. A theoretical rut: revisiting and critically evaluating the generalized under/over-connectivity hypothesis of autism. Developmental science. 2016;19(4):524–49.
-
Ameis SH, Catani M. Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex. 2015;62:158–81.
-
Aoki Y, Abe O, Nippashi Y, Yamasue H. Comparing of white matter integrity betwixt autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol Autism. 2013;4(ane):25.
-
Di X, Azeez A, Li X, Haque Due east, Biswal BB. Disrupted focal white thing integrity in autism spectrum disorder: a voxel-based meta-assay of diffusion tensor imaging studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2018;82:242–viii.
-
Conti Eastward, Calderoni S, Marchi V, Muratori F, Cioni G, Guzzetta A. The offset 1000 days of the autistic encephalon: a systematic review of diffusion imaging studies. Front Hum Neurosci. 2015;9:159.
-
Courchesne E, Karns CM, Davis 60 minutes, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce M, Lord C, Lincoln AJ. Unusual brain growth patterns in early life in patients with autistic disorder an MRI report. Neurology. 2001;57(2):245–54.
-
Ameis SH, Lerch JP, Taylor MJ, Lee Due west, Viviano JD, Pipitone J, Nazeri A, Croarkin PE, Voineskos AN, Lai MC, Crosbie J. A improvidence tensor imaging study in children with ADHD, autism spectrum disorder, OCD, and matched controls: singled-out and not-singled-out white matter disruption and dimensional encephalon-behavior relationships. Am J Psychiatry. 2016;173(12):1213–22.
-
Dimond D, Schuetze M, Smith RE, Dhollander T, Cho I, Vinette S, Ten Eycke K, Lebel C, McCrimmon A, Dewey D, Connelly A. Reduced white matter cobweb density in autism spectrum disorder. Cognitive Cortex. 2019;29(4):1778–88.
-
Frazier TW, Hardan AY. A meta-assay of the corpus callosum in autism. Biological psychiatry. 2009;66(ten):935–41.
-
Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. Journal of Neuroscience. 2010;30(44):14595–609.
-
Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–67.
-
Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;viii(7):333–44.
-
Beaulieu C. The basis of anisotropic water improvidence in the nervous system–a technical review. NMR Biomed. 2002;xv(7-8):435–55.
-
Shemesh NS. Axon diameters and myelin content modulate microscopic fractional anisotropy at brusque diffusion times in stock-still rat spinal string. Front Phys. 2018;6:49.
-
Takahashi 1000, Ono J, Harada K, Maeda Thou, Hackney DB. Diffusional anisotropy in cranial nerves with maturation: quantitative evaluation with diffusion MR imaging in rats. Radiology. 2000;216(iii):881–5.
-
Takahashi One thousand, Hackney DB, Zhang Thou, Wehrli SL, Wright Ac, O'Brien WT, Uematsu H, Wehrli FW, Selzer ME. Magnetic resonance microimaging of intraaxonal h2o improvidence in live excised lamprey spinal cord. Proc Natl Acad Sci. 2002;99(25):16192–6.
-
Song SK, Dominicus SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) improvidence of h2o. Neuroimage. 2002;17(iii):1429–36.
-
Bashat DB, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, Even A, Levy Y, Sira LB. Accelerated maturation of white matter in young children with autism: a loftier b value DWI study. Neuroimage. 2007;37(1):xl–7.
-
Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Botteron KN, Dager SR, Estes AM, Evans AC, Gerig G. White matter microstructure and atypical visual orienting in vii-month-olds at risk for autism. Am J Psychiatry. 2013;170(8):899–908.
-
Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cognitive cortex. 2008;18(eleven):2659–65.
-
Walker Fifty, Gozzi One thousand, Lenroot R, Thurm A, Behseta B, Swedo S, Pierpaoli C. Improvidence tensor imaging in young children with autism: biological effects and potential confounds. Biological psychiatry. 2012;72(12):1043–51.
-
Weinstein G, Ben-Sira L, Levy Y, Zachor DA, Itzhak EB, Artzi M, Tarrasch R, Eksteine PM, Hendler T, Bashat DB. Abnormal white thing integrity in young children with autism. Hum Brain Mapp. 2011;32(four):534–43.
-
Beacher FD, Minati L, Baron-Cohen S, Lombardo MV, Lai MC, Grayness MA, Harrison NA, Critchley Hd. Autism attenuates sex differences in encephalon structure: a combined voxel-based morphometry and improvidence tensor imaging study. American Journal of Neuroradiology. 2012;33(one):83–9.
-
Chiang HL, Chen YJ, Lin HY, Tseng WYI, Gau SSF. Disorder-specific alteration in white thing structural holding in adults with autism spectrum disorder relative to adults with ADHD and developed controls. Hum Brain Mapp. 2017;38(1):384–95.
-
Fishman I, Datko M, Cabrera Y, Carper RA, Müller RA. Reduced integration and differentiation of the imitation network in autism: a combined functional connectivity magnetic resonance imaging and diffusion-weighted imaging report. Annals of neurology. 2015;78(6):958–69.
-
Fitzgerald J, Gallagher L, McGrath J. Widespread disrupted white matter microstructure in autism spectrum disorders. J Autism Dev Disord. 2016:one–xi.
-
Gibbard CR, Ren J, Skuse DH, Clayden JD, Clark CA. Structural connectivity of the amygdala in young adults with autism spectrum disorder. Hum Brain Mapp. 2018;39(3):1270–82.
-
Hong SJ, Hyung B, Paquola C, Bernhardt BC. The superficial white matter in autism and its role in connectivity anomalies and symptom severity. Cerebral Cortex. 2018.
-
Im WY, Ha JH, Kim EJ, Cheon KA, Cho J, Song DH. Impaired white affair integrity and social knowledge in loftier-office autism: diffusion tensor imaging study. Psychiatry Investigat. 2018;15(3):292.
-
Katz J, d'Albis MA, Boisgontier J, Poupon C, Mangin JF, Guevara P, Duclap D, Hamdani N, Petit J, Monnet D, Le Corvoisier P. Similar white matter but reverse grey matter changes in schizophrenia and high-functioning autism. Acta Psychiatrica Scandinavica. 2016;134(1):31–9.
-
Libero LE, Burge WK, Deshpande Hard disk, Pestilli F, Kana RK. White matter diffusion of major fiber tracts implicated in autism spectrum disorder. Brain connectivity. 2016;6(9):691–9.
-
Lin CW, Lin HY, Lo YC, Chen YJ, Hsu YC, Chen YL, Tseng WYI, Gau SSF. Alterations in white matter microstructure and regional book are related to motor functions in boys with autism spectrum disorder. Prog Neuropsychopharmacology Biol Psychiatry. 2019;90:76–83.
-
Lo YC, Chen YJ, Hsu YC, Tseng WYI, Gau SSF. Reduced tract integrity of the model for social advice is a neural substrate of social communication deficits in autism spectrum disorder. J Child Psychol Psychiatry. 2017;58(5):576–85.
-
Nickel M, Tebartz van Elst L, Perlov E, Endres D, Müller GT, Riedel A, Fangmeier T, Maier S. Contradistinct white matter integrity in adults with autism spectrum disorder and an IQ> 100: a diffusion tensor imaging study. Acta Psychiatrica Scandinavica. 2017;135(6):573–83.
-
Samson Ac, Dougherty RF, Lee IA, Phillips JM, Gross JJ, Hardan AY. White matter structure in the uncinate fasciculus: Implications for socio-melancholia deficits in Autism Spectrum Disorder. Psychiatry Res Neuroimaging. 2016;255:66–74.
-
Thompson A, White potato D, Dell'Acqua F, Ecker C, McAlonan G, Howells H, Businesswoman-Cohen S, Lai MC, Lombardo MV, Catani M, MRC AIMS Consortium. Impaired communication betwixt the motor and somatosensory homunculus is associated with poor manual dexterity in autism spectrum disorder. Biol Psychiatry. 2017;81(3):211–nine.
-
Vogan VM, Morgan BR, Leung RC, Anagnostou E, Doyle-Thomas G, Taylor MJ. Widespread white matter differences in children and adolescents with autism spectrum disorder. Journal of autism and developmental disorders. 2016;46(6):2138–47.
-
Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;vii:458.
-
Fingher N, Dinstein I, Ben-Shachar Grand, Haar S, Dale AM, Eyler L, Pierce Chiliad, Courchesne E. Toddlers later on diagnosed with autism showroom multiple structural abnormalities in temporal corpus callosum fibers. Cortex. 2017;97:291–305.
-
Solso S, Xu R, Proudfoot J, Hagler DJ Jr, Campbell K, Venkatraman V, Barnes CC, Ahrens-Barbeau C, Pierce K, Dale A, Eyler L. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol Psychiatry. 2016;79(8):676–84.
-
Wolff JJ, Gu H, Gerig G, Elison JT, Styner K, Gouttard S, Botteron KN, Dager SR, Dawson M, Estes AM, Evans Ac. Differences in white matter fiber tract development nowadays from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169(6):589–600.
-
Xiao Z, Qiu T, Ke X, Xiao X, Xiao T, Liang F, Zou B, Huang H, Fang H, Chu One thousand, Zhang J. Autism spectrum disorder as early neurodevelopmental disorder: prove from the encephalon imaging abnormalities in 2–three years quondam toddlers. J Autism Dev Disord. 2014;44(7):1633–40.
-
Nordahl CW, Simon TJ, Zierhut C, Solomon Yard, Rogers SJ, Amaral DG. Cursory report: methods for acquiring structural MRI data in very immature children with autism without the utilise of sedation. Journal of autism and developmental disorders. 2008;38(eight):1581–90.
-
Werling DM. The role of sex-differential biological science in risk for autism spectrum disorder. Biology of sex activity differences. 2016;7(1):58.
-
Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sexual practice differences in the structural connectome of the man brain. Proc Natil Acad Sci. 2014;111(2):823–8.
-
Tyan YS, Liao JR, Shen CY, Lin YC, Weng JC. Gender differences in the structural connectome of the teenage brain revealed past generalized q-sampling MRI. NeuroImage: Clinical. 2017;15:376–82.
-
Irimia A, Torgerson CM, Jacokes ZJ, Van Horn JD. The connectomes of males and females with autism spectrum disorder have significantly different white matter connectivity densities. Sci Rep. 2017;7:46401.
-
Nordahl CW, Iosif AM, Young GS, Perry LM, Dougherty R, Lee A, Li D, Buonocore MH, Simon T, Rogers S, Wandell B. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Mol Autism. 2015;6(1):26.
-
Zeestraten EA, Gudbrandsen MC, Daly E, de Schotten MT, Catani M, Dell'Acqua F, Lai MC, Ruigrok AN, Lombardo MV, Chakrabarti B, Baron-Cohen S. Sex differences in frontal lobe connectivity in adults with autism spectrum weather. Transl Psychiatry. 2017;seven(4):e1090.
-
Smith SM, Jenkinson G, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise assay of multi-field of study diffusion data. Neuroimage. 2006;31(4):1487–505.
-
Johnson RT, Yeatman JD, Wandell BA, Buonocore MH, Amaral DG, Nordahl CW. Diffusion properties of major white matter tracts in young, typically developing children. Neuroimage. 2014;88:143–54.
-
Lord C, Risi S, Lambrecht 50, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule—generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(three):205–23.
-
Lord C, Rutter M, DiLavore PC, Risi S, Gotham Thousand, Bishop S. Autism Diagnostic Ascertainment Schedule. 2nd ed. Torrance, CA: Western Psychological Services; 2012.
-
Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Periodical of autism and developmental disorders. 1994;24(5):659–85.
-
Gotham Yard, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of autism and developmental disorders. 2009;39(5):693–705.
-
Rutter M, Bailey A, Lord C. SCQ. The Social Advice Questionnaire. Torrance, CA: Western Psychological Services; 2003.
-
Mullen EM. Mullen scales of early on learning (pp. 58-64). Circle Pines, MN: AGS; 1995.
-
Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian assay of neuroimaging information in FSL. Neuroimage. 2009;45(one):S173–86.
-
Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance furnishings and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78.
-
Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans East. Denoising of diffusion MRI using random matrix theory. NeuroImage. 2016b;142:394–406.
-
Veraart J, Fieremans E, Novikov DS. Improvidence MRI dissonance mapping using random matrix theory. Magnetic resonance in medicine. 2016a;76(5):1582–93.
-
Kellner E, Dhital B, Kiselev VG, Reisert Yard. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic resonance in medicine. 2016;76(five):1574–81.
-
Andersson JL, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a not-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage. 2016;141:556–72.
-
Andersson JL, Graham MS, Drobnjak I, Zhang H, Filippini Due north, Bastiani M. Towards a comprehensive framework for motility and baloney correction of diffusion MR images: Within book movement. NeuroImage. 2017;152:450–66.
-
Greve DN, Fischl B. Accurate and robust encephalon image alignment using purlieus-based registration. Neuroimage. 2009;48(1):63–72.
-
Jenkinson K, Smith S. A global optimisation method for robust affine registration of brain images. Medical epitome analysis. 2001;5(2):143–56.
-
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of encephalon images. Neuroimage. 2002;17(2):825–41.
-
Yendiki A, Koldewyn K, Kakunoori South, Kanwisher North, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2014;88:79–90.
-
Solders SK, Cynic RA, Müller RA. White matter compromise in autism? Differentiating movement confounds from true differences in improvidence tensor imaging. Autism Enquiry. 2017;ten(x):1606–20.
-
Smith SM. Fast robust automated encephalon extraction. Hum Brain Mapp. 2002;17(three):143–55.
-
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97.
-
Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98.
-
Mori S, Wakana Due south, Van Zijl PC, Nagae-Poetscher LM. MRI atlas of man white matter: Elsevier; 2005.
-
Jones DK, Knösche TR, Turner R. White affair integrity, fiber count, and other fallacies: the practice's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–54.
-
Badaruddin DH, Andrews GL, Bölte S, Schilmoeller KJ, Schilmoeller G, Paul LK, Chocolate-brown WS. Social and behavioral bug of children with agenesis of the corpus callosum. Child Psychiatry Hum Dev. 2007;38(4):287–302.
-
Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, Abildskov T, Nielsen JA, Cariello AN, Cooperrider JR, Bigler ED. Decreased interhemispheric functional connectivity in autism. Cognitive Cortex. 2010;21(5):1134–46.
-
Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29(48):15089–99.
-
Dean P, Porrill J, Ekerot CF, Jörntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;xi(i):30.
-
Marek South, Siegel JS, Gordon EM, Raut RV, Gratton C, Newbold DJ, Ortega M, Laumann TO, Adeyemo B, Miller DB, Zheng A. Spatial and temporal organization of the individual man cerebellum. Neuron. 2018.
-
Badura A, Verpeut JL, Metzger JW, Pereira TD, Pisano TJ, Deverett B, Bakshinskaya DE, Wang SS. Normal cognitive and social development crave posterior cerebellar activity. eLife. 2018;seven:e36401.
-
Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter Chiliad, Lantos P. A clinicopathological report of autism. Brain. 1998;121(v):889–905.
-
Kemper TL, Bauman ML. The contribution of neuropathologic studies to the understanding of autism. Neurologic clinics. 1993;11(1):175–87.
-
Zeidán-Chuliá F, de Oliveira BHN, Casanova MF, Casanova EL, Noda M, Salmina AB, Verkhratsky A. Up-regulation of oligodendrocyte lineage markers in the cerebellum of autistic patients: prove from network assay of gene expression. Mol Neurobiol. 2016;53(6):4019–25.
-
Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and hereafter directions. International journal of developmental neuroscience. 2005;23(two-3):183–7.
-
Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed past in vivo magnetic resonance imaging. Cognitive Cortex. 2001;eleven(6):490–7.
-
Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, Chen WH. Gender differences and age-related white affair changes of the human encephalon: a diffusion tensor imaging study. Neuroimage. 2008;39(two):566–77.
-
Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage. 2010;52(ane):20–31.
Acknowledgements
The authors would similar to the families and children who participated in the Proceeds and APP studies. We also thank Cory Coleman and Natasha Sharma for their technical assistance and all of the enquiry study staff.
Funding
This research was supported past an Autism Center of Excellence grant awarded by the National Institute of Kid Health and Development (NICHD) (P50 HD093079) as well as the National Institute of Mental Health (R01MH104438 [CWN], R01MH103284 [MS], R01MH103371 [DGA]) and the UC Davis MIND Constitute. This projection was also supported past the Listen Constitute Intellectual and Developmental Disabilities Enquiry Center (U54HD079125). DSA and JKL are supported by the MIND Institute Autism Research Preparation Program (T32MH073124).
Writer information
Affiliations
Contributions
DSA drafted this manuscript and all other authors contributed critical revisions. All authors made substantial contributions to the conception and design of this written report, contributed to aquisition of the information and/or were involved in the assay and interpretation of this data and have approved the concluding version.
Corresponding author
Ideals declarations
Ethics approving and consent to participate
All aspects of the study protocol were approved by the University of California, Davis Institutional Review Board, and informed consent was obtained from the parent or guardian of each participant.
Consent for publication
Non applicable
Competing interests
DGA is on the scientific advisory lath for Stemina Biomarker Discovery and receives consulting fees from Centric Biotherapeutics. All other authors have no competing interests to written report.
Additional information
Publisher's Annotation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Furnishings of Age on Measures of Improvidence. Clusters (Additional file 3: Tabular array S1) of significantly (TFCE p<0.05) increased fractional anisotropy (FA) and decreased mean diffusivity (MD), radial diffusivity (RD), and centric diffusivity (Advert) with age are highlighted. Images are presented in R/50 radiological convention with MNI z coordinates in mm. Skeletonized statistical overlays have been 'inflated' for display.
Additional file 2: Figure S2.
Furnishings of Sex on Measures of Diffusion. Clusters (Boosted file 3: Table S2) showing significantly (TFCE p<0.05) increased partial anisotropy (FA) and decreased hateful diffusivity (MD) and radial diffusivity (RD) in males compared to females across diagnostic groups are highlighted. Images are presented in R/50 radiological convention with MNI z coordinates in mm. Skeletonized statistical overlays accept been 'inflated' for brandish.
Boosted file 3: Table S1.
Clusters with Significant Consequence of Age. Table S2. Clusters with Pregnant Effect of Sexual activity.
Rights and permissions
Open Admission This article is distributed under the terms of the Creative Eatables Attribution 4.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided y'all requite appropriate credit to the original author(due south) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/nothing/one.0/) applies to the information made available in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Andrews, D.S., Lee, J.G., Solomon, 1000. et al. A improvidence-weighted imaging tract-based spatial statistics study of autism spectrum disorder in preschool-aged children. J Neurodevelop Disord 11, 32 (2019). https://doi.org/x.1186/s11689-019-9291-z
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s11689-019-9291-z
Source: https://jneurodevdisorders.biomedcentral.com/articles/10.1186/s11689-019-9291-z
0 Response to "Diffusion Tensor Imaging in Autism Spectrum Disorder a Review"
Postar um comentário